Index:
- Main plant nutrient functions
- Tools for optimal nutrient functions
- Plant nutrients, deficiency symptoms, application rates and methods
- Nitrogen (N) application
- Phosphorus (P)
- Potassium (K)
- Calcium (Ca)
- Magnesium (Mg)
- Micronutrients
4.1 Summary of main plant nutrient functions
|
Nutrient
|
Main functions
|
|
Nitrogen (N)
|
Building block of proteins (growth and yield).
|
|
Phosphorus (P)
|
Cellular division and formation of energetic structures.
|
|
Potassium (K)
|
Transport of sugars, stomata control, cofactor of many enzymes, reduces susceptibility to plant diseases.
|
|
Calcium (Ca)
|
A major building blocks in cell wall and reduces susceptibility to diseases.
|
|
Sulfur (S)
|
Synthesis of essential amino acids cystin and methionine.
|
|
Magnesium (Mg)
|
Central part of chlorophyll molecule.
|
|
Iron (Fe)
|
Chlorophyll synthesis.
|
|
Manganese (Mn)
|
Necessary in the photosynthesis process.
|
|
Boron (B)
|
Formation of cell wall. Germination and elongation of pollen tube.
Participates in the metabolism and transport of sugars.
|
|
Zinc (Zn)
|
Auxins synthesis; enzymes activation.
|
|
Copper (Cu)
|
Influences in the metabolism of nitrogen and carbohydrates.
|
|
Molybdenum (Mo)
|
Component of nitrate-reductase and nitrogenase enzymes.
|
4.2 Tools for optimal nutrient management
The three tools for optimal nutrient management are:
-
Observation of plants and environmental conditions.
-
Soil and water analysis.
-
Leaf analysis.
4.2.1 Observation
Visual symptoms should be used as aids to interpreting soil and leaf analyses:
-
Look for abnormal symptoms in foliage or growth.
-
Look for significant variations in yield.
-
Observation can locate nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, zinc, iron, manganese, copper and boron deficiencies.
4.2.2 Soil analysis
A soil test should be done one year before planting. Soil analysis is performed to assess the need for soil amendment, e.g., lime application to adjust low soil pH, and gypsum application to adjust Ca:Mg ratio or to reclaim alkaline soil. Samples should represent the effective rooting zone. A soil auger may be used to obtain samples. Generally, about 1 liter (1 quart) of soil per sample is adequate. Take 10 or more soil samples of a uniformly looking field of 2 ha (5 acres), and mix the samples. Take more samples for larger plots, or specifically from subplots that look different. Take the samples from the top 20 cm (8 inches). Send them to a soil testing laboratory that can provide recommendations for strawberries. Compare your soil test results with those in Table 4.1.
Table 4.1: Desirable ranges of pH, organic matter, and elements determined by soil test for strawberries (strawberry ph)
|
Parameter
|
Desirable values
|
|
|
pH
|
5.3 – 6.5
|
|
|
Organic matter
|
2% – 3%
|
|
|
|
Available
|
Exchangeable
|
|
Phosphorus
|
67 – 90 kg / ha ; (60-80 Lb / A)
|
|
|
Potassium
|
|
315 – 360 kg / ha ; 280-320 Lb / A
|
|
Magnesium
|
|
280 kg / ha ; (250 Lb / A)
|
|
Boron
|
1.7 – 2.25 kg / ha ; (1.5-2.0 Lb / A)
|
|
|
Zinc
|
11 – 13.5 kg / ha ; (10-12 Lb / A)
|
|
Desirable ranges will vary with soil type (sand, silt, or clay), organic matter already present in the soil, and pH. Soil values may need to be amended in order to correct deficiencies or excesses that are identified.
A pH of around 6.5 is best for sandy textured soils, whereas for finer textured soils (e.g., loam) a pH closer to 5.3 is preferable. Soil pH has a strong influence on nutrient availability, and should be regularly monitored and corrected as needed. A low soil pH (acidic soil) will reduce the availability of nitrogen, phosphorus, potassium, magnesium, and molybdenum, while a high soil pH (alkaline soil) will reduce the availability of zinc, boron, iron, manganese, and copper.
Similar soil tests should be performed several times during the active growth and fruiting seasons. Separate samples should be taken from blocks that differ in age, cultivar type, vine performance, soil type, topography, and fertilizer history. When trying to diagnose a problem with crop growth and yield, samples should be collected from the rooting zones of the worst affected plants. In these circumstances, a second sample taken for comparative purposes from the rooting zones of normal plants may be useful.
4.2.3 Leaf analysis
Intensively grown strawberries require frequent and precise fertility management. Leaf analyses provide the best means of monitoring nutritional status (deficiencies or excesses) and correcting deficiencies that may occur. Leaf analyses not only ensure that yield and quality are optimized, but also protect against applying excess nutrients in the environment, and incurring unnecessary expenses.
The best way to decide how much fertilizer to apply is to collect leaf samples and have them tested for nutrient content. It is recommended to collect tissue samples at first bloom and to continue to do so every two weeks throughout flowering and fruiting.
Nutrient ranges for optimal production are well established. When nutrient levels are outside these ranges, decreases in quality will eventually occur. Tissue analysis can identify nutrient shortages early, before symptoms appear, thus giving growers time to adjust fertilization appropriately.
To collect a tissue sample from strawberry plants, select the most recently mature, trifoliate leaves. These are full-sized, green leaves and consist of a petiole (leaf stalk) with three leaflets. They leaves are usually located 3 to 5 leaves back from the growing point. Avoid collecting damaged leaves. Detach the petioles from the blades as they are collected, but submit them together as one sample.
Each sample should include leaf blades and petioles from 20 to 25 locations within a uniform area. For example, all of the plant material in a single sample should be of the same variety, growing on the same soil type, planted at the same time, and having the same management history. This will serve as a representative sample for that plot.
The most recent mature trifoliate leaf blade sampled at fruiting is the best indicator of the status of N-P-K concentrations in the plant, as well as the secondary elements Ca, Mg, and S, and the micronutrients Fe, Zn, Cu, and B.
The petiole from this same trifoliate leaf sampled throughout the season is the best indicator of the amount of nitrate-nitrogen not yet assimilated in the plant. Petiole analysis is more sensitive to changes in soil nitrate, and the analysis will also be affected by changes in temperature, solar radiation, and soil moisture. A concentration in the petioles in the range 3,000-10,000 ppm nitrate N (on a dry matter basis) indicates an optimal nutritional status.
When submitting leaf samples, an information sheet should be filled out, including fertilization history and environmental conditions. It is particularly important to provide the sampled variety name, as well as its growth stage. Growth stage refers to week of bloom and can be coded B1 through B12 (1st to 12th week of bloom).

Figure 4.1: Proper leaf blade and petiole sampling parts
Source: Clevelend, North Carolina, 2007. https://www.ncagr.gov/paffairs/release/2007/3-07sberry.htm
Leaf blade sampling
Sampling time: During fruiting, preferably at first harvest
Plant part: Leaf blades (excluding petioles)
Collect from: Youngest mature leaves
Quantity per sample: 30-50 units
Comments: Deficiencies are more likely to arise during fruiting, when substantial nutrient uptake is occurring.
Leaf petiole sampling
Sampling time: Throughout the growth season
Plant part: Petioles only
Collect from: Youngest mature leaves
Quantity per sample: ~50
Nutrient concentration ranges for strawberries are listed in Table 4.2.
Table 4.2: Standard concentrations for foliar analysis of strawberries
|
Macro-nutrients
|
Deficient
|
Sub-optimal
|
Optimal
|
Above-optimal
|
Excess
|
|
|
(%)
|
||||||
|
Nitrogen
|
1.5
|
1.8
|
1.9 – 2.8
|
2.9
|
>4.0
|
|
|
Nitrate NO3-N
|
|
|
800 ppm
|
|
|
|
|
Phosphorus
|
0.20
|
0.25
|
0.25 – 0.4
|
0.4-0.5
|
>0.5
|
|
|
Potassium
|
1.2
|
1.3 – 1.6
|
1.6 – 2.5
|
2.5 – 3.4
|
>3.5
|
|
|
Calcium
|
0.6
|
0.69
|
0.7 – 1.7
|
1.7 – 2.0
|
>2.0
|
|
|
Magnesium
|
0.25
|
0.29
|
0.3 – 0.49
|
0.5 – 0.8
|
>0.8
|
|
|
Sulfur
|
0.20
|
0.2 – 0.4
|
0.4 – 0.6
|
0.6 – 0.8
|
>0.8
|
|
|
Sodium
|
|
|
0.10
|
> 0.10
|
|
|
|
Chloride
|
-
|
|
-
|
> 0.50
|
|
|
|
Micro-nutrients
|
(ppm)
|
|||||
|
Manganese
|
40
|
49
|
50 – 200
|
200 – 350
|
>350
|
|
|
Iron
|
30
|
59
|
60 – 250
|
250
|
>350
|
|
|
Zinc
|
15
|
20
|
20 – 49
|
50 – 80
|
>80
|
|
|
Copper
|
5
|
6
|
7 – 19
|
20
|
>20
|
|
|
Boron
|
19
|
24
|
30 – 64
|
65 – 90
|
> 90
|
|
|
Molybdenum
|
0.5
|
|
> 0.5
|
|
|
|
Sources: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au and Ellis et al, Ohio, 2006.; Growing Strawberries, Abdel-Razak, 2004
4.3 Plant nutrients, deficiency symptoms, and application rates and methods
Nitrogen (N)
Nitrogen is the central component of all amino acids, which are the building blocks of all proteins and include, of course, all functional enzymes. Nitrogen is a highly important plant nutrient, which substantially affects quality and yield of strawberries. The plant quickly responds to every positive or negative change in its nitrogen status. Application of nitrogen fertilizers stimulates vegetative growth of leaves, petioles, and shoots. Heavy applications are not recommended because excessive vegetative growth will result in dense leaf canopy that will cover developing fruit, keeping it in a dark, cool and wet microclimate, and enhance the development of fruit rot diseases, such as gray mold. Excessive nitrogen causes fruit softening, which results in easily-damaged strawberries, delayed ripening, decreased yield, and increased powdery mildew and mite pressure.
Nitrogen deficiency is manifested (and consequently visualized more easily) in middle-aged leaves. The yellow strawberry plant leaves occur primarily at middle age, and not when the plant is young, when the new, still-green leaves emerge from the crown. The young leaves emerging from the crown exacerbate the deficient state of the middle-aged leaves by being a stronger sink for the nitrogen that would otherwise have been used in the older leaves. Due to the demand of metabolism and synthesis of the newly emerging foliage, nitrogen will be mobilized from the middle-aged leaves to the new ones. This leads to more severe color changes in the middle-aged leaves, depending on the overall severity of the nitrogen shortage.
Plants with mild N deficiency (N2.0%) have smaller than usual chlorotic older leaves.

Slight nitrogen deficiency on a young leaf (right)
Source: Bolda , 2011; https://ucanr.org/blogs/blogcore/postdetail.cfm?postnum=5404

Advanced nitrogen deficiency on young mature leaves
Source: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au
More severe deficiencies cause shortening of the petioles, which turn red-purple and become brittle. The purple strawberry leaves are attributed to nitrogen and amino acid deficiency (see Figure 4.3). Sometimes, the calyx leaves also turn purple (see Figure 4.4). Nitrogen deficiency also reduces leaf area, root mass, and fruit size.
Frequently, older leaves also become reddish. This should not be confused with N deficiency.

Severe nitrogen deficiency on a single leaf

Severe nitrogen deficiency in the field

Figure 4.4: Nitrogen deficiency – red calyx
Source: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au
The nitrogen available to plants in the soil occurs in two forms: nitrate (NO3-) and ammonium (NH4+). Nitrate is the preferred form for uptake by the plant. It is readily available for use and simple for the plant to metabolize, so nitrate share should be around 90% of the nitrogen applied. However, during colder seasons, the ammoniacal share of the nitrogen may be raised to 25% to 30% because nitrate absorption at these times is relatively inefficient. Prolonged usage of ammonium nitrogen should be accompanied by soil-pH measurements because it can lead to pH-drop, especially, in light soils with low amounts of calcium, which may, in turn, change the uptake rates of other nutrients. Manganese, for example, may reach toxic levels.
Typical nitrogen fertilizers used on strawberries include urea (46% N), ammonium nitrate (34% N), potassium nitrate (13% N), and calcium nitrate (15% N).
Animal manure may be used as a nitrogen carrier because it also contributes to soil fertility via its phosphorus, potassium, and micro-nutrient content. It also improves the physical structure of soil, as well as its hydraulic conductance. The use of animal manure is recommended only if it has been well composted. Although it may contain considerable concentrations of nitrogen at a relatively low price, the risk of microbial contamination by organisms such as salmonella and E. coli is too great for fresh produce. Hence, no fresh manure should be applied to the soil within 120 days before harvest.
In a matted row system, the majority of the nitrogen fertilizer should be applied during the summer months following harvest, when new leaf and shoot (runner) growth is needed to reestablish good planting vigor for the following year’s crop.
During the planting year (assuming the general soil fertility is good), a strawberry planting should receive actual nitrogen at 22 to 45 kg / ha (20 to 40 pounds / acre) incorporated into the soil prior to planting.
Another 35 kg / ha (30 pounds / acre) should be applied in late June to early July.
A final 22 kg / ha (20 pounds / acre) can be applied in late August to early September. Each of these applications corresponds to periods of growth in the plants.
A 1:1 mixture of calcium nitrate Ca(NO3)2 and potassium nitrate (KNO3) with 14% N is the recommended source of nitrogen in new plantings because it is readily available, not volatile, and provides nitrogen, potassium, and calcium.
For established beds, only 11 to 22 kg / ha (10 to 20 pounds / acre) of actual nitrogen, if any, should be applied in the spring.
As part of the renovation process following harvest, 55 to 80 kg / ha (50 to 70 pounds / acre) of actual nitrogen should be applied to the planting, followed by 22 to 33 kg / ha (20 to 30 pounds / acre) in late August to early September.
Table 4.3: Nitrogen fertilizers and their usage indicators
|
Product
|
Analysis
(%N)
|
Development stage for best results
|
Application rate
(kg / 1,000 plants)
|
Comments
|
|
Urea
|
46
|
Early flowering onwards
|
0.4 to 0.5
|
Improves fruit size.
Reduce at fruiting.
Stop if fruit is soft.
|
|
Ammonium nitrate
|
34
|
As for urea
|
0.5 to 0.6
|
Improves fruit size.
Stop if fruit is soft.
|
|
Ammonium sulfate
|
21 +
24% S
|
As for urea
|
0.9 to 1.0
|
Corrosive to mild steel.
|
If nitrogen is applied by nutrigation (through the irrigation system), which is typical in plasticulture systems, pre-plant nutrients should be incorporated into the soil, as recommended above.
In the planting year, actual nitrogen should be applied at 3.5 to 4.5 kg / ha / week (3 to 4 pounds / acre / week) from mid-May through early September.
In the fruiting years, apply approximately 11 kg / ha / week (10 pounds / acre / week) of actual nitrogen from mid-July through late August.
Table 4.4: Nitrogen application rates at annual strawberry plantations in Israel
Source: Abdal-Razak, Israel, 2004
|
Dates
|
Growth stage
|
Actual N application
(kg / ha / day)
|
|
Oct. 1 – Nov. 20
|
Plant establishment and first vegetative growth
|
0.5 – 0.7
|
|
Nov. 21 – Dec. 20
|
1st wave of flowers and fruits
|
1.0 – 1.5
|
|
Dec. 21 – Jan. 20
|
Cold season, slow plant development
|
0.7 – 1.0
|
|
Jan. 21 – Feb. 28
|
2nd wave of flowers and fruits, marked vegetative and reproductive development
|
1.5 – 2.0
|
|
March 1 – March 31
|
3rd wave of flowers and fruits, peak vegetative & reproductive development
|
2.0 – 2.5
|
|
April 1 – end
|
3rd & 4th wave of flowers and fruits, peak vegetative & reproductive development. Plants use mainly stored organic nitrogen.
|
1.0 – 1.5
|
When the grower relies on nitrogen-fixing bacteria to supply part of the nitrogen portion, he should make sure that the soil molybdenum is sufficient, as nitrogen-fixing bacteria cannot fix atmospheric nitrogen to the soil without sufficiently available molybdenum.
Phosphorus is important in the plant's energy management (ADP-ATP) and it plays a role in fruit development. Available phosphorus is especially important during establishment of plantlets after transplanting, for new root formation.
Crude phosphorus is often present in adequate amounts for good strawberry growth, but most of it is not readily available to the plants because it is strongly tied up to both the mineral and organic fractions of the soil. As a result, it does not tend to move through the soil and is not easily leached. Availability is further reduced if the soil pH is too low (5.5), or in the range of 8.0 to 8.5 (Figure 4.5), or if calcium, magnesium, or zinc are present at over-optimal amounts. Soil tests should read 20–30 ppm (Olsen method) for optimal phosphorus uptake. Strawberries do not have a very high phosphorus demand, but if it is not available at optimal rates it can be a limiting factor. Keeping the pH near 6.5 will aid in maintaining the optimal uptake of phosphorus. After planting, it is advisable to monitor phosphorus status by observing the canopy, leaf tissue analysis, and soil tests.

Figure 4.5: The availability of phosphorus to plant roots as a function of the pH of soil solution
Source: Illinois Agronomy Handbook, 1979-80
As a fertilizer, it becomes available to the plants slowly, and it should therefore be worked into the soil prior to planting to improve its uptake throughout the crop's life-cycle.
There is a strong antagonistic relationship between phosphorus and zinc: high phosphorus will invariably reduce zinc uptake, and excess zinc will have the same effect on phosphorus. The ideal phosphorus / zinc ratio is 10:1 respectively.
P deficiency
The first sign of phosphorus deficiency is a deep green appearance of plants and a reduction in leaf size. As the deficiency becomes more severe the upper surface of the leaves develops a dark, metallic gloss, while the underside becomes reddish purple (Figure 4.6). The fruit and fslowers tend to be smaller than normal and the roots are less abundant, stunted and darker. Leaf analysis showing P ≤0.2% in the leaves DM will ascertain this hypothesis.
Most phosphorus should be applied before planting and placed within the root zone. Applying superphosphate after laying plastic mulch in either the planting holes or in the walkways is not effective. Soluble phosphorus fertilizer can be applied by fertigation.

Upper surface of leaves develops a dark metallic gloss
Source: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au

Severe phosphorus deficiency on a leaf, sometimes the underside becomes reddish purple
Cornell University, Department of Horticulture. 2011
Figure 4.6: Phosphorus deficiency symptoms on a strawberry leaf
Table 4.5: Phosphorus fertilizers and their usage indicators
|
Product
|
Analysis
|
Development stage for best results
|
Application rate
(kg / 1,000 plants)
|
Comments
|
|
Triple super phosphate
|
46% P2O5
+
19% CaO
|
Pre-planting
|
2.5 – 3
|
Will supply P for several years.
|
|
Mono-ammonium phosphate (MAP)
|
61% P2O5
+
12% N
|
Early in season and after cutting back for second crop; or before cutting back if plants are kept for a second year.
|
1.0 - 1.2
|
Improves flower and fruit size, and root growth.
|
|
Mono potassium
|
32% P2O5
+
54% K2O
|
Flowering and fruiting
|
0.6 – 0.7
|
Improves flower and fruit size, and root growth.
|
Haifa's specialty phosphate fertilizers
Haifa P™ Nutrigation grade phosphoric acid 0-61-0
Haifa MAP™ Nutrigation grade mono-ammonium phosphate 12-61-0
Haifa MKP™ Nutrigation grade mono-potassium phosphate 0-54-32
Potassium is the third macro-nutrient, (nutritive element required in relatively high amounts) by strawberries. It is an important component of strawberry plants and helps them acquire water by the roots and control water loss by transpiration. Potassium assists in sugar accumulation in the fruit, defies fungal and microbial diseases and insect damage, and plays an important part in tens of enzymatic reactions. Potassium may compete with magnesium for uptake by the roots and must, therefore, be maintained at an appropriate ratio (4:1, K:Mg) in the soil solution to prevent one of these nutrients from overriding the other, thereby creating a deficiency. Soil potassium is sufficient at 120 to 180 ppm.

Figure 4.8: The availability of potassium to plant roots as a function of the pH of soil solution
Source: Illinoi s Agronomy Handbook, 1979-80
Potassium ion (K+) is easily percolated from sandy soil, which is generally the best soil type for strawberries due to their high drainage capacity. It is highly recommended, therefore, to fertilize strawberry fields by continuous small applications of this nutrient throughout the growth season, e.g., by nutrigation. The recommended concentration in the soil solution should be around 1.5 meq / L (=60 ppm).
All major cations: calcium, magnesium, potassium, and sodium compete with each other on absorption by root cells. Therefore, they should be applied to the soil in a balanced manner. Over-liming, for example, can induce a magnesium deficiency, while oversupply of K+ can also reduce magnesium uptake, or can replace calcium in the plant, creating a myriad of problems, and vice-versa. Excessive sodium can replace potassium, producing another set of inherent problems.
The first symptoms of K deficiency (K 1.3%) appear on the upper leaf margins of the older (lower) leaves. The serration tips redden, the injury gradually progressing inwards between the veins until most of the leaf blade is affected.

Figure 4.9: Potassium deficiency on a strawberry leaf, showing increasing severity with age
Source: Strawberry Fertiliser Guide, Primefact 941, 2010. www.industry.nsw.gov.au
This is accompanied almost simultaneously by a symptom which appears to be unique to strawberries. The rachis (extension of the petiole to the central leaflet) darkens and dehydrates, sometimes a necrosis of this leaf region is evident. The blade area on either side of this tissue is similarly affected.

Figure 4.10: Potassium deficiency on a strawberry leaf, darkening and necrosis of leaflet basis and center
Source: Strawberry Deficiency Symptoms, 1980. Ulrich et al, Berkeley, California
Fruit quality is also affected by low potassium levels. The fruit can fail to develop full color, be pulpy in texture and lack flavor.
Figure 4.11: Potassium deficiency on a strawberry fruit. Right fruit is normal, while left fruit grew on a markedly K-deficient plant.
Source: Strawberry Deficiency Symptoms, 1980, Ulrich et al, Berkeley, California
Few runners are produced on K-deficient plants. Those that are produced tend to be short and thin.
The symptoms of potassium deficiency on the leaves can be easily confused with those of magnesium deficiency, see flowing, or with leaf scorch caused by salinity, wind, sun, or dry conditions.
Strawberry fields that are not set up for nutrigation (fertigation) need to be treated with low-solubility potassium fertilizer, such as potassium sulfate as a pre-plant dressing. Better-equipped fields, set up for nutrigation, should usecontinuous low rates of highly soluble potassium fertilizer. Potassium nitrate (13-0-46) is the best choice for this purpose as it contains a high amount of potassium and a marked, though not too high rate of nitrogen. Muriate of potash (KCl) must not be applied to strawberry fields due to its high content of toxic chloride. See Chapter 3, Special sensitivities.
Control
Apply potassium before planting and during early fruit development. A higher rate of potassium should be used in sandy soils and in high rainfall areas. Apply soluble potassium by fertigation after planting.
Table 4.6: Potassium fertilizers and usage indicators
|
Product
|
Analysis
|
Development stage for best results
|
Application rate
(kg / 1,000 plants)
|
Comments
|
|
Potassium nitrate
|
13% N +
46% K2O
|
Flowering
Fruiting
|
0.7 – 0.8
|
Assists in maintaining fruit quality and flavor.
|
|
Potassium sulfate
|
50% K2O
+
16% S
|
Pre-planting
Fruiting
|
0.7 – 0.8
|
As for potassium nitrate
|
|
Mono potassium phosphate (MKP)
|
54% K2O
+
32% P2O5
|
Flowering
Fruiting
|
0.6 – 0.7
|
As for potassium nitrate
|
Haifa's potassium fertilizers for strawberries
Multi-K™ classic - Crystalline potassium nitrate, 13-0-46, for side dressing
Multi-K™ GG - Crystalline potassium nitrate, 13.5-0-46.2, for nutrigation and foliar feeding
Multi-K™ prills - Prilled potassium nitrate, 13-0-46, for side- dressing
Multi™-K pHast - Crystalline low-pH potassium nitrate, 13.5-0-46.2, for side dressing at high soil pH, or high water pH
Multi-npK™ - Crystalline potassium nitrate, enriched with phosphorus, 13-3-43 for all uses.
Haifa MKP™ - Technical grade MKP 0-52-34, for all uses
Calcium is a “secondary” nutrient as classified by strawberry plant requirement rates.
It has many functions in the plant. It is a structural part of the cell walls, by forming cross-links within the pectin polysaccharide matrix. With rapid plant growth, the structural integrity of stems that hold flowers and fruit, as well as the fruit firmness and shelf life, are strongly dependent on calcium availability.
Calcium is also associated with the biosynthesis of proteins and seeds. It assists in root development and movement of carbohydrates within the plant. Calcium insufficiency may impede plant growth and, as its function in root growth is so important, plants may suffer from other nutrient deficiencies as a result of calcium deficiency.
Additionally, many fungi and bacteria invade and infect plant tissue by producing enzymes (e.g., polyglacturonase) that dissolve the middle lamella. Increasing tissue calcium content drastically lowers bacterial and fungal polyglacturonase activity.
Levels of calcium are usually adequate in the soil if the pH is in the appropriate range (6.0-6.2). Soils with a low pH may become calcium deficient. Soil test levels of 1,000 – 1,500 ppm are optimal. Calcium has rather low mobility in the soil and in the plant tissues, where it is mobilized almost exclusively by the transpiration stream.
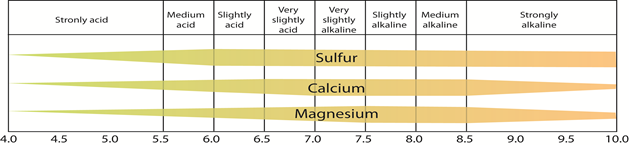
Figure 4.13: The availability of sulfur, calcium, and magnesium to plant roots as a function of the pH of soil solution
Source: Illinois Agronomy Handbook, 1979-80
Calcareous-, sodic-, slightly basic- and neutral soils generally contain a very high rate of soluble and plant-available calcium cations. In such soils, Ca2+ cations also travel freely with the movement of water, and reach plant roots by mass flow. Under these conditions, the amounts of Ca2+ reaching the roots are usually exceedingly higher (by a factor of several hundred) than the amount taken up by the roots, so fertilization with calcium of plants growing in such conditions is generally unnecessary.
Additionally, calcium cations are supplied to the plants by the calcium contained in the irrigation water. Representative values for calcium concentration in irrigation water are in the range of 25 to 200 g / m3 (ppm). With irrigation dose of 5,000 m3 / ha / year, calcium carried with the water to the irrigated plot ranges between 125 and 1,000 kg / ha / year. This is a sufficient rate for strawberries if all this water ends up in the plants' roots zone. But the grower should always make sure that these calculations are valid for his water sources.
Another source of calcium in the soil are phosphate fertilizers such as rock-phosphate (~46% CaO), single superphosphate (~28% CaO) or triple superphosphate (19% CaO), which are sometimes applied at large rates by base dressings, as rich sources of phosphorus. These fertilizers should be worked into the soil to improve uptake.
In acidic soils, however, application of soluble calcium salt, such as calcium nitrate, can be helpful in avoiding aluminum and manganese toxicity. Figure 4.14 shows the positive effect of nutrigation with calcium nitrate on soil pH at the end of the irrigation season, on the surface soil, where it exerted its effect on the soil lattice. It can be assumed that if the calcium were applied at higher rates the increase in pH could be detected at higher depths too.
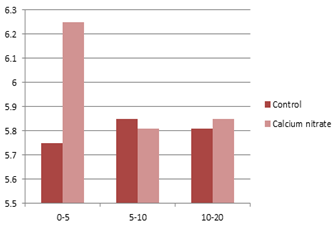
Figure 4.14: Effect of nutrigation with calcium nitrate on soil pH
Source: Haynes, 1990
Soilless cultivation is where calcium application by nutrigation is of highest importance because the inert growth medium cannot supply the necessary calcium. Here, again, calcium cations travel freely within the wetted zone and care should be taken not to leach them off the root zone.
When applying calcium by nutrigation (as well as by all other forms) special attention should be paid to apply it in a balanced way with other cations, such as ammonium, potassium, and magnesium because over-application of any of them will result in subdued uptake of the others, due to competition between the cations on the uptake site at the root level.
The calcium / magnesium ratio is of very high importance in fertility management, as soil structure, nutrient availability, and biological activity are all governed by the relative balance between these nutrients.
The calcium / boron ratio is very important because boron can be toxic in the absence of sufficient calcium. The synergy between these nutrients is delicate, so their deficiencies should ideally be addressed together.
Calcium deficiency
Calcium deficiencies can be the result of a soil fertility problem but are often caused by the plant’s environment, and are associated with periods of rapid growth or soil moisture fluctuations. Young plantings and crops grown in the greenhouse, or on coarse textured, well drained soils and using trickle irrigation, may be subject to possible calcium deficiencies.
Calcium is taken up by the root tips and, once in the plant, is moved within the transpiration stream. It is transported to the most actively transpiring parts of the plant, such as the older leaves. Calcium is not redistributed in the plant, hence it does not move from older leaves to younger leaves like nitrogen. Fruits and young leaves transpire less water or none at all, and symptoms of calcium deficiency first appear in these tissues.
Symptoms include:
-
During rapid leaf growth ‘tip burn’ symptoms may appear on immature leaves. See Figure 4.15. The tips of these leaves fail to expand fully and become black. The tip-burn may show also in folded emerging leaves.
-
Leaf petioles may become necrotic and cause leaf collapse. See Figure 4.16.
-
Expanded young leaves are cupped, puckered, or distorted, with blunt tips.
-
Globs of syrupy liquid may be found on blade midribs.
-
Fruit develops a dense cover of achenes (seeds) in patches or over the entire fruit; it may have a hard texture and acidic taste. See Figure 4.17.
-
The roots become short, stubby, and dark.


Figure 4.15: Calcium deficiency typical tip-burn on leaves

Figure 4.16: Calcium deficiency on leaf petioles of greenhouse strawberry plants

Figure 4.17: Calcium deficiency: small fruit with dense cover of seeds (left), versus normal fruit (right)
Source: Strawberry Fertiliser Guide, Primefact 941, 2010. www.industry.nsw.gov.au
Control
Adjust the soil pH. Apply calcium in the form of agricultural lime or dolomite before planting. Apply calcium nitrate by fertigation or as foliar spray at first sign of deficiency.
Table 4.7: Analysis of calcium nitrate and usage indicators
|
Product
|
Analysis
|
Development stage for best results
|
Application rate
(kg / 1,000 plants)
|
Comments
|
|
Calcium nitrate
|
15.5% N
+
26.5% Ca
|
Post-flowering and during fruit development
|
1.0 to 1.2
|
Improves fruit color and firmness.
Do not mix with magnesium sulfate.
|
Calcium nitrate, CN, Ca(NO3)2 is the most important source of calcium in nutrigation. Once this fertilizer is applied, calcium cations and nitrate anions move in all soils and soilless systems in a similar manner. The plant roots will thus encounter both ion types in similar concentrations within the wetted zone, but it needs to be borne in mind that the nitrate anion is required by the plant at a 10-fold higher rate than the calcium. So the cation nutrition scheme should be balanced with appropriate rates of potassium and magnesium.
Haifa's calcium nitrate fertilizer for nutrigation is marketed under the brand name Haifa Cal™, and features the following parameters
Table 4.8: Haifa's Calcium nitrate (Haifa Ca™l) analysis
|
|
Nutrition grade
|
Greenhouse grade
|
|
Total N
|
15.5%
|
15.5%
|
|
NO3 – N
|
14.4%
|
14.4%
|
|
NH4 – N
|
1.1%
|
1.1%
|
|
CaO
|
26.5%
|
26.5%
|
|
Ca
|
19.0%
|
19.0%
|
|
Insoluble matter
|
2,000 ppm
|
300 ppm
|
Apart from applying calcium nitrate when calcium deficiency symptoms are apparent, it is recommended to:
-
Avoid great fluctuations in soil moisture. Therefore, using dripping irrigation and nutrigation is highly recommended.
-
Adjust nitrogen or crop environment to discourage excessively rapid growth.
-
Calcium deficiency is often confused with:
-
Herbicide injury (such as phenoxy- and dicamba- compounds and sulfur dioxide).
-
Sucking insects e.g., aphids, leafhoppers, cyclamen mites, and plant bugs.
Magnesium is always taken up by plant roots in the form of the divalent cation Mg2+.
Magnesium has many functions in the plant. It is a central structural component of the green chlorophyll molecule, which is responsible for capturing light energy and using it to split the water molecule into its hydrogen and oxygen parts. It is required by a large number of enzymes involved in phosphate transfer. It is also involved in carbohydrate metabolism and movement of carbohydrates from leaves to upper parts; synthesis of nucleic acids. It is related to and stimulates P uptake and transport in addition to being an activator of several enzymes.
The commonly representative concentration of magnesium in plants is around 0.3% of dry weight, as compared with ~3% to 5% for N & K, and 0.5% for P & Ca. Strawberries are no exception to this rule, having 0.30% to 0.50% in dry matter as optimum concentration. Calcareous, slightly basic, and neutral clay soils generally contain a considerable rate of about 6% of magnesium cations in the composition of the clay mineral montmorillonite. While weathering, these soils slowly release the Mg2+ cations to the soil solution. In these soils, Mg2+ cations also travel freely with the water movement, and reach plant roots by mass flow. Under such conditions, the amounts of Mg2+ reaching the roots are usually exceedingly higher (by a factor of several hundred) than the amount taken up by the roots, so fertilization with magnesium of plants growing in these conditions is generally unnecessary. Silt or clay soils with a CEC greater than 10 meq / 100 g soil, are considered to have adequate magnesium if the magnesium share of this CEC is ~10%. Adequate magnesium levels in the soil solution are normally above 50 to 100 ppm.
Additionally, magnesium cations are supplied to the plants by the magnesium contained in the irrigation water. Representative values for magnesium concentration in irrigation water are in the range of 15 to 60 g / m3 (ppm). With irrigation doses of 5,000 m3 / ha / year, magnesium carried with the water to the irrigated plot ranges between 75 and 300 kg / ha / year. This is a sufficient rate for most crops if all the water ends up in the plants' roots zone. But the grower should always make sure that these calculations are valid for his water sources.
In acidic soils, however, application of soluble magnesium salts such as magnesium nitrate can be helpful in avoiding aluminum and manganese toxicity. Figure 4.14 above shows the positive effect of nutrigation with calcium nitrate on soil pH at the end of the irrigation season, on the surface soil, where it exerts its effect on soil lattice. Similar effect can be expected by applying magnesium nitrate.
Soil test levels of 120 to 180 ppm Mg should provide optimal growth for strawberries.
Soilless cultivation is where magnesium application by nutrigation is of highest importance because the inert growth medium cannot supply the necessary magnesium. Here, again, magnesium cations travel freely within the wetted zone and care should be taken not to leach them off the root zone.
When applying magnesium by nutrigation (as well as by all other forms) special attention should be paid to apply it in a balanced way with other cations, such as ammonium, potassium, and calcium because over-application of any of them will result in subdued uptake of the others, due to competition between the cations at the root level on the uptake site. Potassium can compete with magnesium for root uptake, and should therefore be kept at an appropriate balance (4:1, K:Mg) to prevent one from causing a deficiency in the other.
Availability of magnesium is reduced under low soil pH 5.5 (see Figure 4.13), and under heavy use of potassium fertilizers.
Symptoms of Mg deficiency (Mg 0.1%) in strawberry plants are:
-
Tissue in the interveinal regions of older leaves starts with chlorosis, which turns into necrosis as the deficiency progresses.
-
In some instances, a marginal scorch forming a halo pattern can be observed near the base of the serrations on the older leaves.
Marginal leaf scorch begins as yellowing and browning of the upper leaf margin, progressing towards the center of the leaf between the veins (see Figure 4.19). The basal part of the leaf and the short petiole remain green and turgid, unlike in potassium deficiency. Fruits from magnesium deficient plants appear normal, except that they are lighter in color and softer in texture.

Figure 4.19: Magnesium deficiency on strawberry leaves. Marginal scorch (left) and normal leaf (right)
Source: Strawberry Fertiliser Guide, Primefact 941, 2010. www.industry.nsw.gov.au
Control
Deficiencies in strawberry plants can be easily remedied. The most common source of magnesium for pre-planting application is dolomitic or “high-mag” lime, which, in addition to calcium, contains a significant percentage of magnesium. Apply dolomite several months before planting if soil test results indicate low levels of magnesium and low pH.
Magnesium nitrate and magnesium sulfate are soluble magnesium salts that can be applied directly to the soil, or through the irrigation system, and / or be applied to plants as a foliar spray. Magnesium nitrate (Mg(NO3)2; 11-0-0-16MgO) is a fertilizer with extremely high solubility. Its solubility is 8 to 9 folds higher than that of magnesium sulfate. Magnesium nitrate is also relatively rich in nitrate, which is the preferred form of nitrogen for strawberries.
Table 4.9: Magnesium fertilizers and usage indicators
|
Product
|
Analysis
|
Development stage for best results
|
Application rate
(kg / 1,000 plants)
|
Comments
|
|
Magnesium nitrate
|
11% N +
16% MgO
|
Pre-flowering
|
0.3 to 0.5
|
Improves fruit color and firmness.
|
|
Magnesium sulfate (Epsom salt)
|
16% MgO + 14% S
|
Pre-planting
Pre-flowering
|
0.2 to 0.4
|
Improves fruit color and firmness.
Do not mix with calcium nitrate.
|
A foliar spray of magnesium nitrate can also be used to give immediate relief, but it should be tested on a few plants first. Discontinue at the first sign of phytotoxicity.
Haifa produces and markets the most soluble magnesium product available, magnesium nitrate, under the brand nameMagnisal™.
Following are the technical data of Magnisal™:
Analysis
|
MgO
|
16%
|
|
Mg
|
9.5%
|
|
N-NO3
|
11%
|
|
pH (0.1% solution)
|
5.56
|
|
EC (0.1% solution)
|
0.88 dS / m
|
|
Solubility
|
||||
|
0ºC
|
10ºC
|
20ºC
|
30ºC
|
40ºC
|
|
133
|
2,100
|
3,200
|
4,500
|
6,400
|
Magnesium sulfate heptahydrate (Epsom Salt, MgSO4*7H2O; 0-0-0+16.3MgO)
This is a natural occurring mineral with lower solubility. It also contains sulfur, which is a secondary plant nutrient and is hence required by plants at much lower rates. Haifa markets magnesium sulfate heptahydrate under the brand name BitterMag™.
Micronutrients, also termed 'trace elements' are chemical elements present in the plants at 2-4 orders of magnitude less than, e.g., N and K. I.e., while common N and K concentration in dry weight of plants in around 3% to 5%, the common concentrations of micronutrients, is around 5 to 200 ppm.
The most important micro-nutrients in terms of strawberries are boron (B) and zinc (Zn). Others are iron, manganese, copper, and molybdenum.
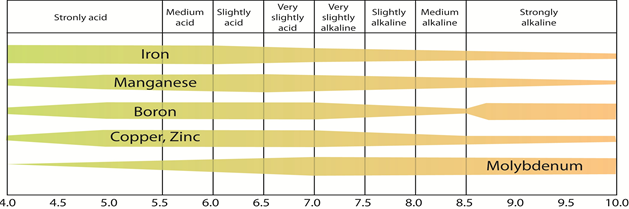
Figure 4.22: The availability of micronutrients to plant roots as a function of the pH of soil solution
Source: Illinois Agronomy Handbook, 1979-80
The cationic micronutrients, i.e., Fe, Zn, Cu, and Mn are highly vulnerable to clay particles in the soil. Therefore, if applied to the soil as inorganic soluble salts, e.g., sulfates, they will normally quickly be immobilized and rendered unavailable to plant roots. Moreover, micronutrients' availability to plant roots is highly pH-dependent. Figure 4.22 above shows that optimal availability of iron, manganese, boron, copper, and zinc takes place in the pH range of 5.0 to 7.5, while molybdenum is most available at pH> 6.5.
Boron
Boron is associated with several of the functions of calcium. Boron improves calcium efficiency and vice-versa, but there is a downside to this system. If calcium levels are too low, boron can become excessive and toxic. Calcium and boron deficiencies should always be addressed together.
Maintaining ideal boron availability for the full crop cycle can prove problematic because boron is the most soluble and leachable of trace elements, and maintaining good levels in wet conditions is a major challenge, especially in light soils. On the other hand, boron availability rapidly declines in dry conditions.
Apart from these soil problems, boron is not readily mobile within the plant. One ppm of boron is considered an ideal level in a soil solution but, as this level is very hard to maintain, well-timed foliar supplementation can be very productive, particularly when applied immediately before flowering.
The functions of boron in strawberry plants:
-
It increases nitrogen availability to the plant.
-
It is involved in the synthesis of cell wall components.
-
Boron increases calcium metabolism and function efficiency within the plant.
-
It has a central role in pollen-tube viability elongation and germination, hence in good seed set and normal fruit development and structure.
-
Boron influences cell mitosis, development, and elongation.
-
It plays a key role in the elongation growth of primary and lateral roots.
-
Boron is important for fruit set.
-
Boron helps in carrying sugars from the leaf to the seeds of the fruit.
Conditions associated with boron deficiencies:
-
Leached, acidic soils.
-
Calcareous or over-limed soils and soils with high pH.
-
Light, sandy soils.
-
Excessive usage of potassium and nitrogen.
-
Drought conditions.
-
Soils low in organic matter (humus is the boron storehouse).
-
Boron performance can be negatively affected by low phosphate levels.
Younger boron-deficient strawberry leaves show puckering and tip-burn, followed by marginal yellowing and crinkling with reduced growth at the growing point. See Figure 4.23 below.


Figure 4.23: Boron deficiency on strawberry leaves
Source: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au
Moderate deficiency of boron reduces the flower size (Figure 4.24) and decreases pollen production, resulting in small, ‘bumpy’ fruit of poor quality (Figure 4.25). Root growth can be stunted.

Figure 4.24: Boron deficiency on strawberry flowers, left - normal, right - B- deficient
Source: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au

Figure 4.25: Boron deficiency on strawberry fruit
Source: Strawberry fertilizer guide, Primefact 941, 2010. www.industry.nsw.gov.au
Control
It must be borne in mind that excessive amounts of boron are toxic to the plant and should not be used excessively. The deviation from optimal concentrations of boron in the plant can risk its performance in both a deficiency or an excessive concentration, and the difference between the two is rather small, so care must be taken to make sure that the plant has enough, but never too much. No more than 500g / ha (1 pound / acre) of actual boron should be applied to a field in one year.
Preventing boron deficiency can be achieved by adding borax to the soil before planting. Taking care of a newly expressed boron deficiency in the field is done by applying a foliar spray, or fertigating with a soluble boron compound such as:
-
Boric acid (H3BO3) with 17% B.
-
Inkabor/Solubor (20.5% B).
-
Ulexite: A calcium / borate product containing 14% boron, naturally fused with a calcium synergist.
-
Stabilized boron humates: This product helps in the management of boron nutrition, mainly because complexing the boron by humates minimizes the leaching problem.
Borax is a series of salts of boric acid that differ in their hydration states (and therefore in their B content). It normally has 11% boron, with poor solubility, but it is useful in maintaining soil levels by pre-planting application.
Zinc (Zn)
The functions of zinc in soil and plant nutrition:
-
Zinc activates many enzymes.
-
Zinc has a critical role in the production of the auxin IAA, which regulates plant elongation and expansion as well as fruit development.
-
It plays an important role in the formation and activity of chlorophyll.
-
It is involved in protein synthesis.
-
It is important for carbohydrate metabolism.
-
Zinc plays a major role in the absorption of moisture. Plants with adequate zinc nutrition have enhanced drought-handling capacity.
The optimal level of zinc in strawberry leaf tissue is 25 to 45 ppm.
Common reasons for reduced zinc availability to strawberry plants:
-
Zinc in the soil is more available to plants under low pH conditions. Therefore, reduced zinc availability to plants is very common in calcareous soils. Likewise, heavy applications of lime and / or phosphorus reduce the availability of zinc to the plants.
-
Soils lacking mycorrhizal fungi.
-
Light, sandy soils.
-
High phosphorus levels – phosphate ties up zinc.
-
Cold, wet soils.
-
Soils featuring anaerobic decomposition, i.e., zinc bonds with sulfides produced in these conditions and becomes insoluble.
Zinc deficiency is easily distinguished on strawberry leaves by the discolored ‘halo’ that develops along the serrated margins of young, immature leaf blades. As the leaves continue to grow the blades become narrow at the base and eventually become elongated with severe deficiency. Yellowing and green-veining occurs. See Figure 4.26.
The fruit size may appear normal, but the number of fruit is reduced.


Figure 4.26: Manifestation of zinc deficiency by discoloration along the serrated margins of leaf blades, and chlorosis of interveinal area.
Source: Strawberry Fertiliser Guide, Primefact 941, 2010. www.industry.nsw.gov.au
Control
Zinc fertilizers
-
Zinc oxide (ZnO) is a viable option to build soil levels and contains 80% zinc. However, it is usually a slow-release product that will only become available towards the end of the crop.
-
Zinc sulphate heptahydrate (ZnSO4*7H2O) contains 23% zinc, while monohydrate (ZnSO4*1H2O) contains 35%. In terms of cost per unit of zinc, zinc sulphate monohydrate is usually the most economical choice. Add zinc sulfate (ZnSO4) with 36% Zn to the fertilizer program and apply at planting to soils known to be low in zinc. It is typically applied as part of a fertilizer blend to the soil, or applied as a foliar spray to help achieve uniform distribution of a small amount of material over a relatively large area.
-
The most efficient way to treat zinc deficiency is by Zn Chelate; 14% EDTA-Zn.
Chelates are generally highly water soluble. Chelates can be pre-dissolved and injected into the trickle irrigation system as liquid, for precise application commanded by the small amounts required. The chelating agent guards the micronutrient from immobilization as long as it is in the soil solution. But once it gets close to the acidic root surface it is released from the chelate complex, enters the plant and wanders around in it in the form of cation-organic acid, e.g., Zn-citrate.
Foliar spray. The application of zinc by fertigation or as a foliar spray can give immediate relief. However, the use of zinc sulfate as a foliar spray may damage young leaves, flowers, and fruit. Discontinue treatment at the first sign of phytotoxicity.
Haifa's products for controlling zinc deficiency
Haifa Micro™ Zn EDTA 14%, a stable, water-soluble and non dusting zinc EDTA chelate
Multi-K™ Zn crystalline potassium nitrate enriched with zinc
Multi-K™ ME 13-0-45+ME (crystalline)
Iron (Fe)
Iron is the most abundant element in the known universe, and yet the lack of plant-available iron can be a serious yield-limiter in almost every area of agriculture. Most soils contain 20 to 200 MT of iron per hectare, but very little of this reserve is plant available. Also, potential iron problems are magnified, as iron does not move easily within the plant. Ideal iron soil analysis ranges between 40 and 200 ppm, but levels exceeding these figures do not guarantee avoidance of Fe deficiency.
The functions of iron
-
Iron is one of the essential elements required for biological nitrogen fixation.
-
Adequate iron, in plant-available form, is essential for protein synthesis. non-haeme iron proteins.
-
Fe is an indispensable oxygen-carrier for chlorophyll production.
-
Iron is a constituent of cytochromes, it is involved in respiratory linked dehydrogenases.
-
Iron is also involved in the reduction of nitrates and sulfates and in reduction processes by peroxidase and adolase.
-
Fe increases leaf thickness, which, in turn, enhances nutrient flow, which eventually increases yield.
-
Iron makes the leaf darker, with a greater capacity to absorb solar energy.
Conditions creating iron deficiencies
-
Excessive phosphate applications or high phosphate levels in the soil.
-
High manganese, copper, or molybdenum reduces iron uptake. Iron deficiency is bound to take place when manganese exceeds iron levels in the soil solution by two-fold or more. Therefore, iron should always be higher than manganese to avoid likely iron lockup.
-
Cold, wet conditions limit iron uptake, particularly in the early growth stages.
-
Excessive lime applications reduce iron availability.
-
Inadequate soil aeration hinders Fe mobility.
-
High soil pH (7.5 or higher, see Figure 4.22) and / or calcareous and poorly drained soils.
-
Low organic matter, because organic matter serves as a chelating agent for Fe and other trace elements.
Iron deficiency is first expressed by yellowing of the leaf blade while veins retain their green color. As the deficiency becomes more severe, yellowing increases to the point of bleaching and the leaf blades turn brown. See Figure 4.28. Fruit size and quality are not greatly affected.


Figure 4.28: Iron deficiency symptoms on strawberry leaves. Green network of fine veins distinct in early stages, followed by interveinal yellowing and then severe chlorosis
Control
Check soil pH. If the pH level is high, cease liming and use pH-reducing fertilizers such as ammonium sulfate.
Apply Fe Chelates, e.g., Fe-EDTA (13% Fe), or Fe-DTPA (7% Fe), by fertigation or by foliar spray, when symptoms first appear. The type of chelating agent recommended depends mainly on the pH value of the soil or growth medium, and on the crop. If iron sulfate (FeSO4), an inorganic soluble salt of iron, is applied to the soil, it will normally quickly be immobilized and rendered unavailable to plant roots.
Haifa's products for controlling iron deficiency
Haifa Micro™ Fe-EDDHA 6% water soluble chelate
Haifa Micro™ Fe-EDTA 13% water soluble chelate
Multi-K™ ME crystalline potassium nitrate enriched with micro-nutrients
Foliar applied Fe chelates are most effective in correcting crop deficiencies in the growing season and are perhaps the best solution for strawberries grown on high-pH soils. Lowering the pH of clay-like soils to increase the availability of Fe does not make economic sense, and may even be nearly impossible.
Manganese (Mn)
Manganese is critically important in the reproductive stage of plant growth. The common requirement for soil-solution manganese is 30 to 100 ppm.
The functions of manganese
-
It is involved in production of amino acids and proteins.
-
Hastens the fruiting and ripening of crops.
-
Accelerates and improves seed formation and germination, and the early establishment of the seedling.
-
It is a critical enzyme activator for dehydrogenases, decarboxylases, kinases, oxidases, peroxidases, and non-specifically by other divalent cation-activated enzymes.
-
Essential for carbohydrate and nitrogen metabolism.
-
Manganese has important roles in chlorophyll formation and nitrate reduction.
-
Required for chlorophyll production.
-
Manganese is a vital component of photo-system II in the photosynthesis process. Hence, it's required for the assimilation of carbon dioxide in photosynthesis.
-
Directly involved in plant uptake of iron.
Conditions creating manganese deficiencies
-
Soil pH >8, or 4.5. See Figure 4.22.
-
Soils with very high levels of organic matter.
-
Light, sandy soils.
-
Natural excessive calcium or overliming can tie up manganese, even moderate applications of lime will magnify a manganese deficiency.
-
High phosphorus and iron can limit manganese uptake.
-
An overuse of potassium and magnesium can reduce manganese uptake because of soil pH increases.
-
When combined sodium and potassium base saturation percentages total over 10%, then manganese uptake will be limited, regardless of soil test results. This problem occurs most often in lighter soils.
-
Cool, wet conditions.
The first sign of manganese deficiency is pale greening to yellowing of young leaves. As the deficiency progresses, the main veins remain dark green, while the interveinal areas become yellow, followed by scorching and upward turning of the leaf blade margins.
Scorch areas advance towards the centre of the leaf as broad rays extending across the veins. The fruit size can be reduced (see Figure 4.30). In many cases, the leaf blade develops a network of dense yellow dots (see Figure 4.31).

Manganese- deficient strawberry leaf
Somewhat faint interveinal chlorosis beginning at margins and progressing towards midrib

Manganese- deficient strawberry plant
Source: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au
Figure 4.30: Manganese deficiency symptoms on strawberry leaves

Figure 4.31: Manganese deficiency symptoms on strawberry leaf – a network of dense yellow dots
Control
Manganese sulfate (MnSO4) contains 31% manganese and can be used as a soil supplement or through fertigation when deficiencies are slight (i.e., 20 to30 ppm of Mn2+ in soil solution). It is generally not cost effective to build soil levels with manganese sulfate. In more seriously deficient soils the product of choice is Mn-EDTA (13% Mn) applied by fertigation or by foliar spray.
Foliar spray of manganese sulfate can give some relief but may be toxic, especially at flowering and at fruit-set.
Success in using Mn-EDTA for fertigation and foliar sprays is generally guaranteed.
Haifa's products for controlling zinc deficiency
Haifa Micro™ Mn-EDTA 13% water soluble chelate
Multi-K™ ME crystalline potassium nitrate enriched with micro-nutrients
Manganese toxicity may take place when soil-pH drops during relatively short periods of time, as a result of continuous use of ammonium fertilizers.
Copper (Cu)
The ideal range of copper concentration in the soil solution is 5 to 10 ppm. Excessive copper can affect phosphate, zinc, and iron uptake. Where copper levels in soils exceed several hundred ppm, copper can become the yield limiting factor in many crops, including wheat, corn, cotton, pasture, canola, orchard crops, and vegetables, including onions, spinach, and brassicas.
The functions of copper
-
Essential in many enzyme systems, particularly those associated with respiration (many oxidase enzymes). Copper is also a constituent of cytochrome oxidase and haeme in equal proportions. Anaerobic metabolism, and hormonal metabolism.
-
Important for water movement within the plant.
-
Copper is a key component in many proteins.
-
Essential for chlorophyll formation and photosynthesis processes.
-
Cu-proteins have a marked effect on the formation of lignin, and the composition of cell walls. Hence it is important for stems strength, flexibility, and elasticity.
-
Vitally important for root metabolism.
-
Helps prevent development of chlorosis, resetting, and die-back.
-
Provides a natural fungicidal effect. This feature can be a problem where copper levels in the soil have become too high due to extensive human sprays (above 15 ppm), as this fungicidal quality becomes detrimental to beneficial fungi in the soil.
Conditions creating copper deficiencies
-
Soil pH >8, or 4.5, see Figure 4.22.
-
Light, sandy, coastal soils are invariably deficient.
-
Peaty, high-organic matter soils tend to hold copper, strongly reducing plant availability.
-
Excessive phosphate and nitrogen can limit copper availability.
-
Over liming can create deficiencies.
-
High zinc levels can reduce copper uptake.
-
Drought conditions can intensify any copper problems.
Copper deficiency symptoms
Copper is relatively immobile within plants, so deficiency symptoms normally occur first on new growth, and youngest leaves are worst affected.
Copper-deficient strawberry leaves develop chlorotic to bleached blades, especially on their bases. Their veins remain green, but may sometimes become brownish-black. See Figure 4.33.

Figure 4.33: Copper deficiency symptoms on strawberry leaf
Control
When copper deficiency is encountered, copper sulfate, CuSO4 (25% Cu) may be an efficient and inexpensive solution, and it is the best material to build soil levels. 20 kg/ha of copper sulfate is the maximum application rate for soil-grown strawberries, at any one time, as copper applications can affect soil-fungi. This figure should be reduced 20-fold in case of soilless grown strawberries.
CuSO4 can also be used as a foliar feeding at 0.5%, to address deficiencies. It was proved that it is more effective than the more expensive hydroxides and oxychlorides.
The most effective way of correcting Cu deficiencies in strawberries is by applying copper chelates such as Cu-EDTH (14% Cu), by nutrigation (fertigation) or by foliar sprays.
Multi-K™ ME, is a product containing potassium nitrate plus a mixture of all micronutrients, for safe continuous maintenance of the plantation.
Haifa's products for controlling copper deficiency
Haifa Micro Cu-EDTA 14% water soluble chelate
Multi-K™ ME crystalline potassium nitrate enriched with micro-nutrients
Molybdenum (Mo)
Molybdenum is the least abundant of all the recognized micro-nutrients in the soil. Molybdenum is the only trace element whose availability increases as pH rises.
The functions of molybdenum
-
Essential for nitrogen fixation.
-
Required for the synthesis and activity of the enzyme nitrate-reductase (reduces nitrates to ammonium in the plant).
-
Involved in electron transport in plant metabolism.
-
Linked to organically-bound phosphorus uptake in the plant.
Conditions associated with molybdenum deficiencies
-
Acidic soils that are highly leached.
-
Timber soils.
-
Acidic, sandy soils.
-
Soils that are high in other metal oxides.
Symptoms of deficiency
In strawberries, Mo deficiency looks very much like nitrogen deficiency (paleness and stunting). The leaf edges also tend to burn because of the accumulation of unused nitrates.
Control
When Molybdenum deficiency is encountered, molydbic acid monohydrate (MoO3·H2O (59.6% Mo), or sodium molybdate dihydrate Na2MoO4·2H2O (39.7% Mo), can be applied.
Haifa-Micro™ Comb is a stable, water-soluble and non-dusting balanced mixture of metal EDTA-chelates,. including molybdenum as follows: 7.1% Fe, 3.48% Mn, 1.02% Zn, 0.76% Cu, 0.485% Mo.
To sum up this chapter of nutritional disorders in strawberries, here is a short manual.
Table 4.10: Visual symptoms of nutritional disorders of strawberries
Source: Strawberry fertiliser guide, Primefact 941, 2010. www.industry.nsw.gov.au
|
Leaf symptoms
|
Possible causes
|
|
Uniform yellowing
|
Nitrogen or sulfur deficiency or poor soil drainage
|
|
Yellowing with veins remaining green
|
Zinc, manganese or iron deficiency
|
|
Yellowing of leaf base
|
Copper deficiency
|
|
Darkening of leaf base or center vein
|
Potassium deficiency
|
|
Dark green foliage
|
Phosphorus deficiency
|
|
Leaf scorch
|
Potassium or magnesium deficiency or salt toxicity
|
|
Growing points damaged with restricted growth
|
Calcium or boron deficiency
|
|
Brown-black veins
|
Copper or boron deficiency
|
|
|
|
|
Fruit symptoms
|
Possible causes
|
|
Poor pollination (bumpy fruit)
|
Boron deficiency, frost damage or high temperature during flowering
|
|
Hard seed
|
Calcium deficiency
|
|
Soft, poor color and flavor
|
Potassium deficiency
|
Need more information about growing strawberries? You can always return to the strawberry fertilizer program and you can also visit our iron deficiency in plants and soils!





